WHO WE ARE
Company
Cellaïon is a Belgian biotech company developing Life saving advanced therapies to repair tissues and regenerate the liver, Cellaïon targets life threatening diseases.
We are located in the biotech valley of the Walloon region in Belgium.
We are a team of biotech entrepreneurs, physicians and highly talented scientists in the field of liver diseases and cell based regenerative medicine.
Our strong IP base is issued from our own research and the academic research at UCLouvain, developed with the objective to generate new products.
Our lead product HepaStem® is in advanced clinical development in Acute-on -Chronic Liver Failure (ACLF), a life threatening condition affecting more than 100,000 patients worldwide each year, with a 50% mortality.
The liver has an immense capacity to regenerate, but this process is often overwhelmed by the ongoing disease and by the tissue inflammation, with functional breakdown of the organ and impaired regenerative process.
We at Cellaion want to restore a favorable environment to reboost the regenerative process, and ensure a second life to the native liver instead of loosing it.
HepaStem® has a unique anti-inflammatory, immunomodulatory and regenerative capacity and is administered by a simple intravenous infusion.
TEAM
Management



TEAM
Board of Directors

Philippe POULETTY
Etienne SOKAL, M.D., Ph.D.

Claire COROT

Philippe DE BACKER, Ph.D.

Caroline THIELEN

Fouzia LAGHRISSI-THODE
Walid M. Abi-Saab, M.D.
OUR SCIENCE
What we do
We repair tissues and regenerate organs using cell signaling technology.
Our first target is the liver space. The liver is the master organ of the body, and loss of its function causes failure of other vital organs and death.
Cellaïon aims to stop progression of chronic liver disease, control inflammation, stop fibrous tissue accumulation and eventually allow organ recovery and regeneration.
Our lead compound HepaStem® acts as a therapeutic cargo, delivering to the liver and inflamed tissues appropriate immunomodulatory signals with specific anti-inflammatory, anti-fibrotic and regenerative activities.
Our treatment covers the wide spectrum of liver diseases, from the highly life-threatening Acute-on-Chronic Liver-Failure (ACLF) and Acute Alcoholic Hepatitis, to intermediate severity acute decompensation of cirrhosis (AD) and progressive chronic liver diseases caused by Non-Alcoholic Steatohepatitis (NASH).
Genetic modification of HepaStem® allows to address specific targets in selected diseases.
Thanks to our allogeneic platform our of the shelf product and our multiple patients can be treated from one single tissues sourcing.
More than 100 patients have already received HepaStem®, easily administered by simple peripheral vein infusion, and the very high safety profile is now established with more that 10 years follow up.
Our proof of concept trial in ACLF is ongoing in many European countries and will be further extended in the US.
Reference:
ACLF
Acute-on-chronic liver failure
Acute-on-Chronic Liver Failure (ACLF), a well characterized clinical entity affecting patients with chronic liver disease including cirrhosis, and characterized by a sudden deterioration of the liver, followed by other vital organs such as kidney, brain, lung or heart. ACLF has mortality from 42% up to 75% at 3 months.
NASH
Non-alcoholic Steatohepatitis
Progressive Non-Alcoholic Steatohepatitis is the most severe manifestation of Non-alcoholic fatty liver disease (NAFLD). NASH is closely related to the triple epidemic of obesity, hyperlipidemia and diabetes type 2. The fat chronic deposits can induce liver injury and a sustained inflammation. NASH can progress to more serious disease stages, such as advanced fibrosis, cirrhosis or liver cancer.
AAH
Acute Alcoholic hepatitis
Acute Alcoholic Hepatitis is the inflammation of the liver caused by drinking excessive amounts of alcohol. The disease can be sudden with a high mortality rate.
AD
Acute Decompensated cirrhosis
Decompensated Cirrhosis is defined as an acute deterioration in liver function in a patient with cirrhosis and is characterised by jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome or variceal haemorrhage. Patients with AD can develop ACLF.
OUR SCIENCE
For Who
We care for the liver & we care for lives
The prevalence of CLD is more than 1.5 billion worldwide and cause more than 2 million deaths per year.
In European countries, the median cirrhosis prevalence was 833/100,000 (range 447-1100)(*)
The major and end complications of CLD are cirrhosis (1.2 million deaths) and liver cancer (790,000 deaths) – account for 3.5% of all deaths worldwide.
There is currently no approved treatment for these patients and only liver transplantation is considered as a last and unique hope.
PIPELINE
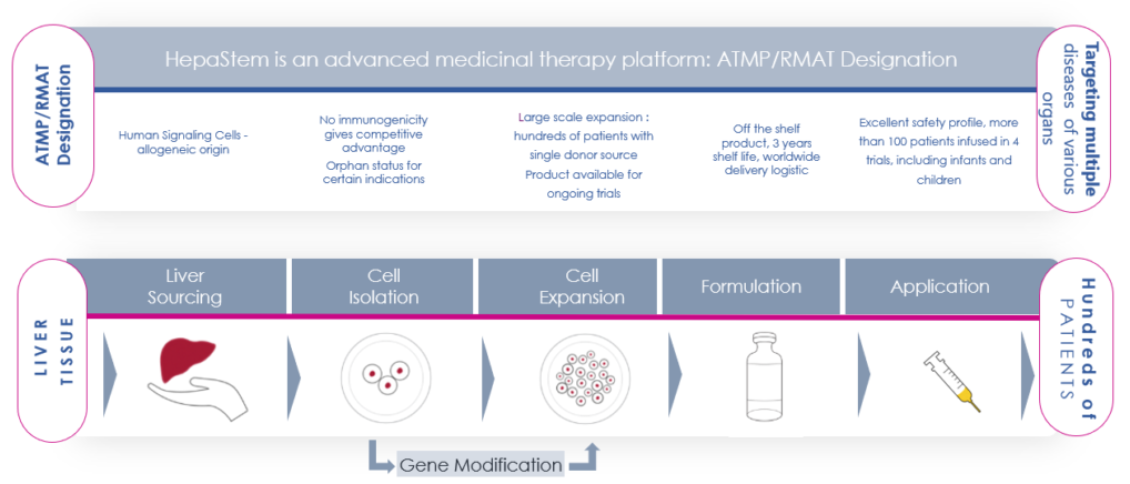
Our product: HepaStem®
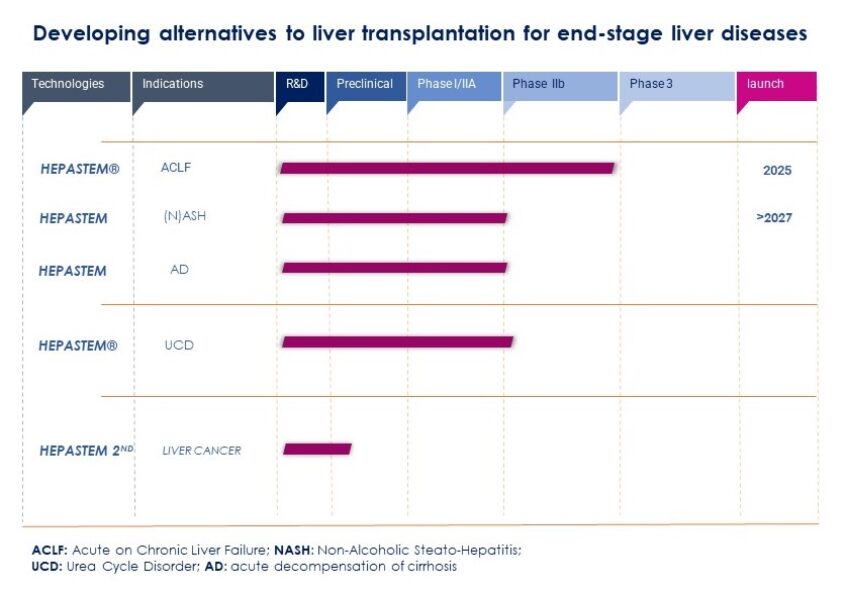
HepaStem® platform.
PIPELINE
Clinical Trial
Publication:
A phase II study of human allogeneic liver-derived progenitor cell therapy for acute-on-chronic liver failure and acute decompensation.
Published in April 2021 in JHEP Liver

PIPELINE
Manufacturing
PARTNERSHIP
Collaborative projects
Current partnership
Cellaïon as partner